
Matter is a substance made up of different types of particles that occupy physical space and have momentum. This means that matter must have both volume and mass. Protons, neutrons, and electrons are common examples of matter. The properties of matter can be divided into two groups, namely physical properties and chemical properties. Substances can be further characterized by their composition as elements, molecules, or compounds.

Figure 1: A, B, and C are all substances. A is a monatomic element. B is a molecule and an element. C is a compound.
Read on to find out why this can be a difficult topic for teachers and students, suggestions for handling the situation, and ideas for why virtual labs can make things easier.
There are three reasons in particular why properties of matter can be difficult, even for the most diligent of students
The physical and chemical properties of matter occur at the subatomic level, you cannot see it. Not being able to visualize the process and not seeing its relevance in the real world can undermine learning and prevent students from staying motivated.
Properties of matter: Physical properties are properties of a substance that can be observed or measured without changing the substance's chemical composition. Chemical properties are the ability or inability of a substance to combine or change into other substances. The ability of a substance to change depends on the chemical composition of the substance.
Conductivity
Conductivity is a physical property of a material that describes how resistant the material is to the flow of electric current. Conductive materials allow current flow (low resistance/resistance). Examples are metals and graphite, which have free electrons that are free to flow in the material, while non-conductive materials offer resistance to electrons flowing. Collisions between atoms and electrons disrupt the current and release some of the energy as heat. Because of this, electrical circuits and devices may become hot after prolonged use.

Figure 2: Electrons traveling through a wire made of a conductor and an insulator.
Reactivity
In the fourth period of the periodic table, the general trend of reactivity decreases, but why is that? An atom is reactive when it is easy to remove electrons for a chemical reaction. When an atom is smaller, its outer electron is closer to its positive nucleus, which means it takes more energy to remove it. The larger the atom, the greater the distance between the nucleus and the electrons, which means it can be moved more easily, and therefore the atom is more reactive. We also see that the general trend in atomic size decreases over time, which provides the reason for this correlation. Period 4 metal reactivity series and atomic size
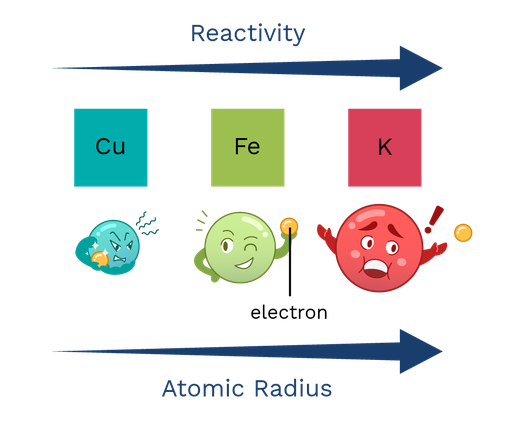
Figure 3: As the atomic radius increases, the reactivity increased
Correlation vs causality
Correlation is a pattern between two variables, but without evidence that one causes the other. If there is a provable explanation for one variable that affects another, the correlation is causal, but without explanation, the correlation may not be causal. For example, there is a relationship between hot summer days and the sale of ice cream, but also between the sale of ice cream and umbrellas. The first example is casual because when the weather is nice, more people go out and enjoy the sun and then buy ice cream. The second example is not causal because selling umbrellas does not cause a decrease in ice cream sales or vice versa; both relate to time. Correlation can even be random when there is no relationship at all between variables.
With these points in mind, here are five things you can consider introducing into the physical and chemical properties of matter lessons to make them more interesting, approachable, and enjoyable to teach for you and to learn for your students.
In 1800, John Dalton, an English chemist, physicist, and meteorologist, determined that each chemical element consists of a unique type of atom and that atoms have different masses. He proposed a system of chemical symbols and, after examining the relative weights of atoms, arranged them in a table.
He also proposed the atomic theory.
A material is flammable if it can be used as a fuel to release energy in the chemical reaction of combustion. We need to add heat and oxygen to the fuel for this chemical reaction to occur. Without these materials, combustion is incomplete and eventually dies. A good example of combustion is a wood fire, where wood acts as a fuel and the heat of the fire is the energy released in the reaction.
Flammability describes the difficulty in using the material as a fuel for combustion. A substance is flammable if it can be easily ignited with a single spark or at low temperatures, e.g. paper or hydrogen gas. These substances must be handled with care. The combustion triangle shows the material to be burned
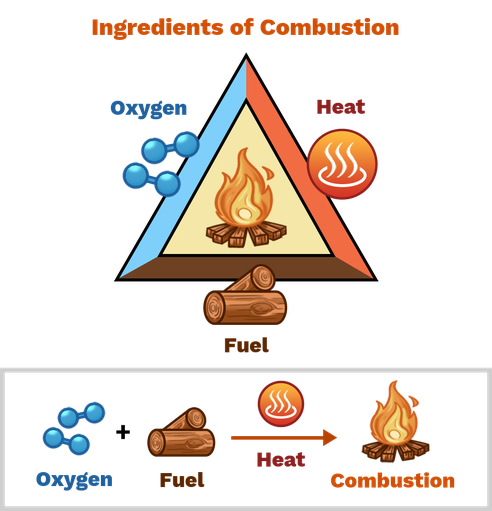
Figure 4: Combustion requires oxygen, fuel, and heat.
Compressive strength:
Although both are composed entirely of carbon atoms, brittle graphite and strong diamond have very different strengths. All these physical properties are due to structure. Diamond has a large covalent lattice structure, a regular pattern of strong covalent bonds in a tetrahedral shape. These strong connections and strong form make a Diamond a very strong structure. Due to its compressive strength, it is particularly suitable for mining machinery.
In graphite, the carbon forms a 2D layer in the form of hexagonal tessellation linked by covalent bonds. These layers are loosely connected by intermolecular bonds that break more easily, making graphite very brittle. This makes graphite useful in pencils. Graphite breaks down as you write, leaving marks on the page.
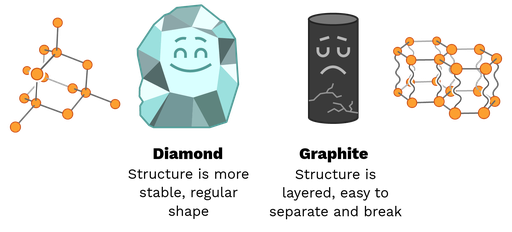
Figure 5: The structure of the diamond is what makes it strong. The structure of graphite is what makes it weak
Visualizations can be a huge help in appreciating this topic, there are a few ways to get around it but with the use of memory aid such as Combustion requires HOF namely
H - Heat
O - Oxygen, and
F - Fuel.
A unique way to teach the physical and chemical properties of matter is through a virtual laboratory simulation. At Labster, we’re dedicated to delivering fully interactive advanced laboratory simulations that utilize gamification elements like storytelling and scoring systems, inside an immersive and engaging 3D universe.

Check out the Labster physical and chemical properties of matter simulation that allows students to learn about the properties of matter through active, inquiry-based learning. In this simulation, students will go on a mission to learn about conductivity, flammability, reactivity, and compressive strength. Putting all this knowledge together, you must make a space suit that can survive a fire tornado.
Learn more about the physical and chemical properties of matter simulation here or get in touch to find out how you can start using virtual labs with your students.

Labster helps universities and high schools enhance student success in STEM.
Request DemoRequest a demo to discover how Labster helps high schools and universities enhance student success.
Request Demo