
Many experiments involving chemicals require their use in solution form. This means that two or more substances are mixed in known quantities. This may involve weighing an accurate amount of dry matter or measuring an accurate amount of liquid. Accurate solution preparation increases experiment safety and chances of success.
To prepare a solution containing a given concentration of a substance, it is necessary to dissolve the desired number of moles of solute in sufficient solvent to give the desired final volume of solution.
Read on for some thoughts on why this can be a startling topic for teachers and students, five suggestions for changing it, and thoughts on why a virtual lab could be of benefit to you.

Figure 1: An image showing solution preparation setup in the lab. It is available in the solution preparation simulation from Labster. The simulation is usable for High School and University/College courses.
There are 3 main reasons why students are overwhelmed with solution preparation topics. Recognizing this problem is the first step in making the topic more accessible.
Solutions are made to react at a molecular level that students cannot see. Not being able to visualize the process and not seeing its relevance to the real world can frustrate learning and make it difficult for students to stay motivated.
Mass and mole conversions: The relationship between mass and quantity in moles for any chemical substance is determined by its molar mass, which is defined as the mass of 1 mole of the pure substance expressed in grams. Symbols and units: The following notation is often used:
mass [g]= m
amount [mol]= n
molar mass [g/mol] = M
Example 1
NaCl (table salt) has a molar mass of 58.44 g/mol. What is the mass of 2.10 mol NaCl?
m(NaCl) = n(NaCl) * M(NaCl) = 2.10 mol * 58.44 g/mol= 122.7 g
Example 2
H2O (water) has a molar mass of 18.02 g/mol. What is the amount of water (in mol) equivalent to 4.3 g?
n(H2O) = m(H2O) / M(H2O)= 4.3 g / 18.02 g/mol = 0.239 mol.
Volumetric flasks are standard glassware in chemistry laboratories. Its main goal is to create solutions with high concentration accuracy. Volumetric flask filled with liquid.
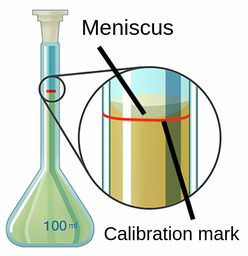
Figure 2: Volumetric flasks
The volumetric flask is made of clear glass and has a wide base that tapers towards the neck. The neck of the pumpkin is long and narrow with straight sides. There is a horizontal red line on the neck of the bottle called the calibration mark. The arrows indicate that the liquid fills the flask only up to the mark. It is often equipped with a top stopper which can be used to completely seal the bottle concerning mixing the contents. Since there is only one calibration mark on the flask, students cannot determine the volume of its contents with reasonable accuracy until it is filled to the mark.
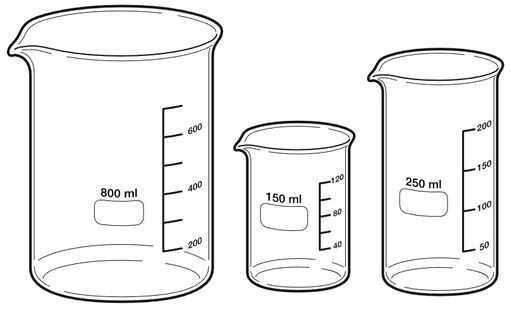
Figure 3: Beakers
Beakers are good for temporarily storing solutions or solvents while working in the lab. It's easy to add and transfer liquids, but you can't measure volume very accurately. Three beakers with different volumes, 800 milliliters, 150 milliliters, and 250 milliliters. All beakers are made of clear glass. They are cylindrical in shape with straight sides and a small spout at the top. The beaker has a horizontal line evenly spaced on one side to measure the volume of liquid inside.
With those points in mind, here are five things you can incorporate into a solution preparation class to make it more engaging, accessible, and fun for you and your students.
The main purpose of a bulb pipette, also known as a volumetric pipette, is to transfer very precise volumes from one container to another. Typical sizes range from 1-100mL. Usually, to fill and dispense liquid from a volumetric pipette, students use a pipette bulb or commonly called a pipette helper.
A measuring cylinder is also known as a graduated cylinder. It is excellent for relatively accurate measurement of liquid volumes, usually with an error of less than 1%, and is very commonly used in laboratories.
A blue-capped bottle is often used to store solvents or liquid solutions. They come with different types of caps depending on what you are using.
Transparent bottle with a blue plastic cap. The bottle is labeled with a capacity of 250 milliliters.
Steps to use an analytical balance: An analytical balance is a high-precision laboratory balance used to determine an object's exact mass. Objects can be solids, liquids, granules, or powders.
Remove any item from the analytical balance
Place the empty draw cup on the scale, close it and click the tare to reset.
Open the balance, take that weighing dish and place it on the workbench.
Using a spatula, pour the solids from the container into the weighing dish.
Place the weighing dish with the substance on balance, and close it to make an accurate reading.
Solute: A substance, usually a solid, that is dissolved in a solution, which is usually a liquid.
Solvent: This is usually a liquid substance capable of dissolving other substances.
Solution: A homogeneous mixture of two or more substances (often present as a liquid).
Reagent: A substance that can react with another substance or an agent which can produce a chemical reaction.
Standard solution: A solution whose strength or concentration is known.
The concentration or strength of a solution can be expressed in several ways namely:
Normal solution: A solution containing 1 gram equivalent of solute in one liter of solution. It is denoted by ‘N’.
Molar concentration: The concentration of a chemical or compound in solution is often expressed in moles per liter or moles/L. This is also known as molarity and so M is often used instead of moles/L. Molarity is found by dividing the amount in moles by the volume of the solution in liters:
C = n / V
where C is the molarity or molar concentration, n is the number of moles, and V is the volume in liters.
Molal solution: A solution that contains one mole solute dissolved in a Kilogram of solvent. It is represented by ‘m’
Chemicals used in laboratories can be toxic, flammable, corrosive, and a host of other hazards. Handling chemicals safely is an important part of ensuring the health and safety of those who work with them. It also saves on disposal and production costs and is healthier for the environment. When working with chemicals, it is always important to know what properties one is working with and what hazards can be associated with them. Let's look at some safety considerations:
Always wear appropriate personal protective equipment (PPE) when handling chemicals and preparing solutions.
Read the chemical label twice before use.
When using concentrated chemicals to make a solution, be sure to slowly add the more concentrated solution to the less concentrated one.
A unique way to teach solution preparation is with virtual lab simulations. At Labster, we are dedicated to providing fully interactive state-of-the-art lab simulations that use gamified elements such as storytelling and scoring systems in an immersive, 3D world.
Explore Labster's solution preparation simulation, which allows students to learn about solution preparation through active inquiry-based learning. In the simulation, students embark on steps involved in preparing an aqueous solution of a given molarity from ammonium chloride – a water-soluble salt, from start to storage.

Learn more about solution preparation simulations here or contact us to find out how you can use the virtual lab with your students.

Labster helps universities and high schools enhance student success in STEM.
Request DemoRequest a demo to discover how Labster helps high schools and universities enhance student success.
Request Demo