
Electrostatics is the branch of physics that deals with the study of electrical charges at rest. In physics, the charge is known as an electric charge. It is the property of matter that experiences a force when it is placed in an electromagnetic field. The charge has two types: positive charge and negative charge. Both types have the same number of charges but consist of opposite signs.
An atom contains a positive charge when it has more protons than electrons. In the same way, when an atom contains more electrons than protons, it has a negative charge.
An inverse square law states that a physical quantity reduces with an inverse square of the distance from the source. An example of inverse square law is Coulomb’s law.
Coulomb’s law states that the magnitude of the electrostatic force of attraction or repulsion is directly proportional to the product of the magnitudes of the charges and inversely proportional to the square of the distance between two charges.
F = k. (Q1) . (Q2) / r2
In the above equation, F is the force of attraction and repulsion between two charged bodies, (Q1) and (Q2) are the electrical charges, k is the proportionality constant, and r is the distance between the two charge bodies.
Sometimes, students may find some difficulties while learning Coulomb’s law. At Labster, we compiled all the reasons that make this topic difficult to understand. We also explain five methods to make this topic easier for students. In the end, we will convince you why a virtual lab simulation is important for students as well as for teachers to convey the topic of Coulomb’s law in the class.

Figure: An image shows the charge interaction between the positive and negative charge from Labster virtual laboratory of Coulomb’s law.
Discover Labster's Coulomb's Law virtual lab today!
There are three main reasons that make Coulomb’s law a difficult topic, even for hardworking students.
Many abstract concepts are used in Coulomb’s law. These are charges, electrostatic force, electrons, and protons that students cannot see with the naked eye. It is hard for students to believe in abstract concepts. During studying this topic, they cannot imagine the structure of electric charges which makes this topic difficult to learn.
Some students may experience difficulties while learning Coulomb’s law because it’s content-heavy. The topic consists of basic law, electrostatic forces, examples, formulas, and limitations that can be hard for students to remember. So, students may lose interest in reading Coulomb’s law due to the complexity of the topic.
In Coulomb’s law, you have to solve different numerical problems by using the formula. Also, it is important to know about the graph representation of the electrostatic force of attraction and repulsion between the charges. Students may find it confusing and boring to solve the numerical and make a graph to represent Coulomb’s law.
We are familiar with the difficulties that students experience during studying Coulomb’s law. So, there are five methods that make this topic simpler and more interesting to understand.
Charles Augustin de Coulomb:
Charles Augustin de Coulomb was a French physicist who provided a mathematical explanation of the two electrically charged bodies. He was the first person who studied electrostatics. In 1785, he introduced an equation that shows the force causing two bodies to attract or repel each other. It is known as Coulomb’s inverse square law or Coulomb’s law. He represented the three reports related to electricity and described the laws of attraction and repulsion between the charges and bodies. He developed a device known as “dubbed the torsion balance”. It helped to measure the charges and estimate the force of attraction and repulsion between the charges.
Students need to learn the interesting facts and properties of Coulomb’s law. The electrostatic force is expressed through the unit Newton. Electrostatic force is a vector quantity that has both magnitude as well as direction. The direction of the electrostatic force depends upon the charge of the particles, whether they are similar or opposite. It is necessary to know that the electrostatic force is not constant. The electrostatic force is the main reason that explains the distance between the charges.
Coulomb’s law represents accurate information about the force present between the two bodies. Coulomb’s first law states that two objects with the same charges repel each other while two objects with different charges attract each other. Coulomb’s second law states that the force of attraction or repulsion between two objects is directly proportional to the magnitude between the objects and inversely proportional to the square of the distance between two objects.
There is a new constant in Coulomb’s law known as the permittivity of free space. It is also called as permittivity of the vacuum.

Figure: An image explaining Coulomb’s law from Labster theory
Coulomb explains the properties of the electric force of the charge at the rest state. These are the following:
Limitations of Coulomb’s law: Coulomb’s law has four main limitations. Students should know the limitations of Coulomb’s law that help them to understand the topic properly.
Students find Coulomb’s law more interesting when they learn real examples related to the topic. They can perform these experiments in class to know that Coulomb’s law is really applicable in daily life.
Students will enjoy learning Coulomb’s law when they see the color diagrams. The diverse colors make it easier to differentiate between the charges and force of interaction between the objects.
The color image used below represents the charge interaction between the objects. Students will understand better about charge interaction by seeing this virtual image. For example, the two positive charges always repel while a positive and negative charge object always attracts. If learners see a color diagram explaining this concept, it will be better for them to understand it.
Virtual lab simulation is an advanced way to teach students about tough topics like Coulomb’s law. At Labster, we provide a 3D laboratory simulation with gamification elements like storytelling and a scoring system. It enhances the interest of students in the topic and clears their concepts.
Labster Coulomb’s law simulation explains the basic law, electrostatics forces, inverse square law, data collection method, magnitude and direction of the force, and graph representation. A virtual lab simulation is effective for students to learn Coulomb’s law and for teachers to convey the topic appropriately.
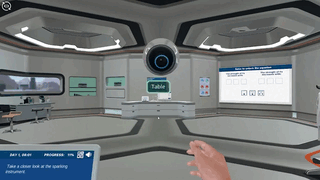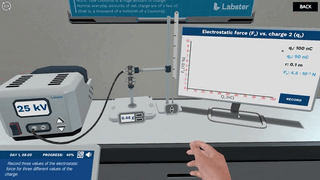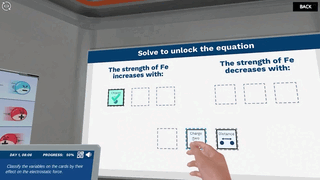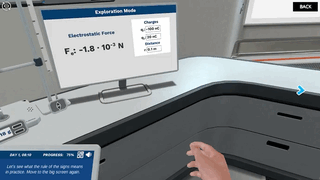
Try our free 30-day All Acess Educator's Pass today and play the Coulomb's Law simulation alongside 300+ other virtual labs!

Labster helps universities and high schools enhance student success in STEM.
Request DemoRequest a demo to discover how Labster helps high schools and universities enhance student success.
Request Demo